

There are five types of mammalian Ig heavy chains denoted by Greek letters: α, δ, ε, γ and μ. The type of heavy chain defines the overall class or isotype of an antibody. Learn more about the advantages of F(ab) and F(ab')2 fragments. Fc fragments are often used as Fc receptor blocking agents in immunohistochemical staining. Often, because of their smaller size and lack of cross-linking (due to the Fc region's loss), F(ab) fragments are radiolabeled in functional studies. Fragmenting IgG antibodies is sometimes useful because F(ab) fragments (1) will not precipitate the antigen, and (2) will not be bound by immune cells in live studies because of the lack of an Fc region. Also, dye and enzymes can be covalently linked to antibodies on the Fc portion of the antibody for experimental visualization.Īntibody fragments have distinct advantages in specific immunochemical techniques. The Fc fragment provides a binding site for endogenous Fc receptors on the surface of lymphocytes and secondary antibodies. The F(ab) regions contain the variable domain that binds to cognate (specific) antigens. The Y-shape of an antibody can be cleaved into three fragments by the proteolytic enzyme pepsin: two F(ab) regions and an Fc region. V H – heavy chain variable domain, V L- light chain variable domain, C H – heavy chain constant domain, C L – light chain constant domain. The base of the antibody includes constant domains (C). Antigen binding occurs at the variable domain (V), consisting of immunoglobulin heavy (H) and light chains (L). This region is essential for the function of the antibody during an immune response.įigure 1. Antibody structure. The Y-shaped antibody is joined in the middle by a flexible hinge region. The antibody base consists of constant domains (C) and forms the fragment crystallizable region (Fc). This region binds tightly to a specific part of an antigen called an epitope. The top of the Y shape contains the variable region (V), also known as the fragment antigen-binding (F(ab)) region. Each Y unit contains two identical copies of a heavy chain (H) and two identical copies of a light chain (L) heavy and light chains differ in their sequence and length. Antibodies specifically bind unique pathogen molecules called antigens.Īntibodies exist as one or more copies of a Y-shaped unit composed of four polypeptide chains (Fig. We recommend using normal serum with these antibodies to prevent the binding to Fc receptors.Guide to the structural components that make up an antibody - heavy chains, light chains, F(ab)/Fc regions - and antibody isotypes.Īntibodies, also known as immunoglobulins (Ig), are large, Y-shaped glycoproteins produced by B-cells as a primary immune defense.
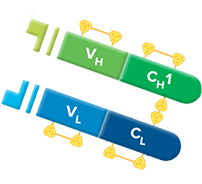

However, as opposed to F(ab) fragments, F(ab') 2 fragments can both bind and precipitate antigens thanks to their two binding sites. The use of F(ab') 2 fragments also avoids unspecific binding to Fc receptor on live cells or to Protein A/G.į(ab') 2 fragments are not recommended for blocking since they have two binding sites that are available to capture the primary antibody introduced subsequently. F(ab') 2 fragmentsĭivalent antibody fragments (F(ab') 2 fragments) are smaller than whole IgG molecules and enable a better penetration into tissue thus faciliting better antigen recognition in IHC. These antibodies are not recommended for blocking immunoglobulins in WB and ELISA. Monovalent antibody fragments (F(ab) fragments) are powerful tools to block background from primary antibody binding and in double staining experiments.į(ab) fragments are used to block endogenous immunoglobulins on cells, tissues and exposed immunoglobulins in multiple labeling experiments using primary antibodies from the same species.Īfter the blocking step with normal serum, we recommend incubating F(ab) fragments in excess to block endogenous immunoglobulins in IHC. Using fragment secondary antibodies F(ab) fragments View our F(ab') 2 fragment secondary antibodies View our F(ab) fragment secondary antibodies As fragment antibodies do not have Fc portions, they do not interfere with anti-Fc mediated antibody detection.Penetrate tissues more efficiently due to their smaller size.Eliminate non-specific binding between Fc portions of antibodies and Fc receptors on cells (such as macrophages, dendritic cells, neutrophils, NK cells and B cells).Figure legend: The light chain (LH) folds into a variable domain (VL) and a constant domain (CL) whereas the heavy chain is composed of one variable domain (VH) and three (IgG and IgA or four constant domains (IgE).Īdvantages of fragment secondary antibodies


 0 kommentar(er)
0 kommentar(er)
